
Click on Image to view full size
| Table of Contents |
Archived Issues |
Search | Image Directory |
<< Issue |
>> Issue |
April 2000 • Volume 16 • Number 3
Current Concepts
Biodegradable Implants in
Sports Medicine: The Biological Base
Andreas Weiler, M.D. [MEDLINE LOOKUP]
Reinhard F. G. Hoffmann, M.D. [MEDLINE LOOKUP]
Andreas C. Stähelin, M.D. [MEDLINE LOOKUP]
Hanns-Joachim Helling, M.D. [MEDLINE LOOKUP]
Norbert P. Südkamp, M.D. [MEDLINE LOOKUP]
Sections
| Abstract | TOP |
Summary: Biodegradable implants are increasingly used in the field of operative sports medicine. Today, a tremendous variety of implants such as interference screws, staples, sutures, tacks, suture anchors, and devices for meniscal repair are available. These implants consist of different biodegradable polymers that have substantially different raw material characteristics such as in vivo degradation, host-tissue response, and osseous replacement. Because these devices have become the standard implant for several operative procedures, it is essential to understand their biological base. The purpose of this report is to provide a comprehensive insight into biodegradable implant biology for a better understanding of the advantages and risks associated with using these implants in the field of operative sports medicine. In particular, in vivo degradation, biocompatibility, and the osseous replacement of the implants are discussed. A standardized classification system to document and treat possible adverse tissue reactions is given, with special regard to extra-articular and intra-articular soft-tissue response and to osteolytic lesions.
| (Click on a term to search this journal for other articles containing that term.) |
| Key Words: Biodegradable
implants, Clinical
application, Sports
medicine, Biocompatibility,
In
vivo degradation |
Materials that disintegrate in the body have been emerging over the past 3 decades, and
there are now numerous implants available in the fields of orthopaedic surgery, general
surgery, maxillofacial surgery, cardiology, gynecology, and urology. Terms such as
absorbable, resorbable, and degradable, with or without the prefix ‘bio’ are
inconsistently used in the literature. We use the term biodegradable to characterize
materials that show disintegration after implantation and subsequent complete excretion.
For many years, biodegradable implants have been thought to offer advantages over metal
analogs. In orthopaedic practice, metal implants can distort magnetic resonance imaging
(MRI),1,2 and
they release metal ions into the surrounding tissue. Further disadvantages include the
need for a second surgical procedure for implant removal and complicated revision surgery
resulting from the presence of the implant. The intent of biodegradable implants is to
provide secure initial fixation strength while allowing degradation and replacement by the
host tissue. Therefore, there is no need for implant removal, revision surgery is not
compromised, and radiological imaging is not distorted. In addition, functional loads can
be assumed earlier by the healing bone while the material is degrading.3,4
In sports medicine, the development and use of biodegradable implants has emerged late
compared with other fields, such as general orthopaedics, orthopaedic trauma surgery, and
maxillofacial surgery. However, the strong interest of joint surgeons in these materials
has led to the development of numerous implants becoming available and, as a result, the
market has shown a dramatic change within the last few years. Today, we can choose from a
large variety of biodegradable implants, such as sutures, staples, tacks, anchors,
interference screws, and devices for meniscal repair. High mechanical properties of a
biodegradable implant may be of primary importance in fracture fixation or other
orthopaedic procedures where the implant is exposed to high loads. This may explain the
slow progress of biodegradable implant technology in this field. In contrast, as several
clinical and biomechanical studies have shown, certain operative procedures in sports
medicine do not require implants of high mechanical strength. For interference screw
fixation in cruciate ligament reconstruction, the cancellous bone may be the weak link and
not the interference screw.5-7
The fixation strength of a suture anchor construct may be limited by the suture or the
bone stock quality.8,9
Biodegradable implants consist of different polymeric raw materials that have
substantially different material characteristics and tissue response. We believe that it
is inappropriate to apply the term biodegradable to all these different materials.
Furthermore, it is important to know the basic biology of these materials, such as in vivo
degradation, osseous replacement, and biocompatibility, in order to evaluate their
appropriateness for the use in operative sports medicine. The purpose of this review is to
focus on current developments and to provide the clinician with an insight in
biodegradable implant biology.
| IN VIVO DEGRADATION | TOP |
Today, approximately 40 different biodegradable polymers are known.10,11 Of these, the
following materials have been studied to be used in orthopaedic implants:
Additionally, composite materials consisting of PLLA/tricalcium phosphate or
PLLA/hydroxyapatite have been introduced.12-15 Of major interest in implant technology in the
field of operative sports medicine are the poly--hydroxy acids such as PLLA and PGA
including their copolymers and stereocopolymers.16
In principal, synthetic biodegradable polymers consisting of poly--hydroxy acids undergo
an unspecific hydrolytic chain scission due to water uptake.17
Degradation starts at the amorphous phase of the implant leading to fragmentation of the
material to smaller parts, which are phagocytosed primarily by macrophages and
polymorphonuclear leukocytes.18-20 Polymeric lactic acid oligomers degrade to
monomers which enter the Krebs cycle and get dissimilated to carbon dioxide and water.17 Beside the hydrolytic chain scission, glycolic
acid monomers can be released by unspecific esterases and carboxypeptidases.21
Degradation kinetics of different raw materials differ substantially, which may be
attributable to the hydrophilic or hydrophobic nature of the different polymers.
Furthermore, although the degradation kinetics of biodegradable implants depend primarily
on polymer choice, a large variety of additional factors also appear to contribute to this
process, including molecular weight, sterilization, implant size, self-reinforcement, and
processing techniques.11,22-30
We know that in vitro hydrolysis testing could differ markedly from in vivo testing
because of the additional influence of environmental conditions. Due to a possible
interaction between degrading polymers and the healing tissue, the in vivo degradation
characteristics of biodegradable implants should be known. Unfortunately, only a few
studies have investigated the in vivo degradation of the different polymers used in
biodegradable implants, and these have reported vastly different results because of
inconsistent test conditions and different implant processing techniques.11 Vert et al.31
tested the tensile strength of different polylactides implanted in sheep tibiae. They
reported that PLLA maintains its tensile strength for over 150 weeks. In contrast, Gerlach
et al.24 found that PLLA rods lose
approximately 50% of their bending strength within 4 weeks if implanted in rat dorsal
muscles. Fischer et al.14 reported that 2-mm
rods made of PDLLA implanted in rat dorsal muscles maintained 90% of their initial bending
strength for over 6 weeks with subsequent rapid degradation. In contrast, Mainil-Varlet et
al.32 reported that pushout forces of PDLLA
rods implanted in sheep tibiae increased continuously over a period of 6 months and were
significantly higher than those of PLLA rods. This may be the result of the implant
swelling caused by water uptake of the stereocopolymer. In principal, it is reasonable to
assume that slow or intermediate degrading materials such as PLLA, PLLA-co-PDLLA, or PDLLA
maintain their mechanical strength at least for the time required for proper tissue
healing. Other materials, such as PDS, PGA, PGA-co-TMC, or PDLLA-co-PGA, which are
expected to degrade more quickly, could suffer a significant loss of mechanical strength
in vivo within the period of tissue healing. However, clinical studies have not yet
reported any healing failure resulting from the use of these materials.33-39 For long-,
intermediate-, and slow-degrading interference screws, different animal studies have
proven that these screws withstand the forces until the graft is incorporated.40-43
While most reports studied the degradation kinetics of biodegradable implants by measuring
strength retention biomechanically, less is known about the long-term fate of implant
remnants in the body. Pistner et al.30 found
a large amount of particles of block-polymerized and injection-molded PLLA implants in
dorsal rat muscle tissue 112 weeks after implantation, although the material had lost 80%
of its bending strength 32 weeks after implantation. Clinical reports have shown that
remnants of high molecular-weight PLLA implants could still be found several years after
implantation. Bergsma et el.44 found implant
remnants up to 5.7 years after stabilization of midface fractures with PLLA plates and
screws.44 Böstman et al.45 described the necessity of partial implant removal
up to 45 months after stabilization of ankle fractures with highly crystalline
self-reinforced PLLA screws. The occurrence of late hydrolytic degradation may depend on
the degree of the material's crystallinity. Twelve months after implantation of
self-reinforced PGA rods, Weiler et al.46
found an absence of birefringent material at the implant site, but crystalline PGA
remnants were detected in lymph nodes for up to 24 months after implantation (Fig 1).
| Figure 1. Inguinal lymph node of a
sheep 6 months after implantation of crystalline self-reinforced PGA pins. Macrophage with
intracellularly deposited polymeric particles (black arrows). (Reprinted with permission.46) |
 |
Click on Image to view full size |
At rearthroscopy, Stähelin et al.36 found bulky remnants of a highly crystalline PLLA interference screw 20 months after implantation (Fig 2).
| Figure 2. Bulky fragments
of a highly crystalline PLLA interference screw 20 months after implantation compared with
a nonused specimen. (Reprinted with permission.36) |
 |
Click on Image to view full size |
These reports suggest that a complete degradation of highly crystalline, so-called biodegradable, implants does not occur within an appropriate time. To monitor the complete degradation process of synthetic biodegradable implants in bone tissue, Pistner et al.47 introduced a scheme of 5 phases of degradation (Table 1).
| Phase | Tissue Reaction |
| 1. Healing phase | Unchanged implant, development of a fibrous capsule with a high amount of fibroblasts |
| 2. Latency phase | Unchanged implant, fibrous capsule gets thinner with less cells and more fibers or direct implant contact to bone |
| 3. Protracted resorptive phase | Mainly central degradation of the implant, development of cracks, mild to moderate cellular response with invasion of macrophages and foreign-body giant cells |
| 4. Progressive resorptive phase | Progressive disintegration of the implant with a severe tissue response (macrophages, foreign-body giant cells) |
| 5. Recovery phase | No polymer remnants detectable, development of scar tissue or osseous replacement of the former implant site |
| OSSEOUS REPLACEMENT | TOP |
A major intent of biodegradable implants is complete tissue replacement at the former
implant site. Although an early replacement with fibrous granulation tissue takes place
during degradation,46,48-53 less is known about the long-term fate of the
former implant site and its osseous replacement. Although a complete osseous replacement
has been anticipated for all biodegradable implants, it has not yet been shown either
experimentally or clinically in most cases. To facilitate uncompromised revision surgery,
a complete osseous replacement should occur within a 2- to 3-year time frame to allow for
a second interference fit or tack fixation as, for example, in cruciate ligament and
shoulder revision surgery.
The osteogenic reaction of the host tissue starts early after implantation of the
polymeric material and shows an osseous enclosure within the first few weeks51,53 (Fig 3).
During or following implant degradation, osseous replacement may follow 3 different patterns:
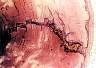
| Figure 4. Bone trabeculae
growing into a PLLA-co-PDLLA pin 15 months after intramedullary implantation in a sheep
tibia. |
 |
Click on Image to view full size |
| Figure 5. New bone
trabeculae growing in the center of the former implant site 6 months after implantation of
self-reinforced PGA pins in a sheep distal femur. The tetracycline fluorescence (black
arrows) indicates the osseous activity. There are implant remnants left (white arrows).
(Reprinted with permission.46) |
 |
Click on Image to view full size |
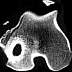
| Figure 6. CT scan showing
severe osseous sclerosis of an implant site 18 months after metaphyseal implantation of
PLLA-co-PDLLA pins in a sheep. |
 |
Click on Image to view full size |
In general, it is reasonable to assume that the faster a material degrades, the earlier
the osseous replacement takes place (Figs 8 and 9).36,54
Materials such as PDLLA-co-PGA, PLLA-co-PDLLA, or PDLLA are considered to degrade faster compared with PLLA implants, for which the degradation process has been described to last for several years.44,55,56 To our knowledge, no single report has shown complete osseous replacement of a PLLA implant in a clinical or experimental setup (Figs 10 and 11).
Several experimental studies have been performed to investigate tissue response and tissue replacement after implantation of PLLA material into bone.27,49,52,53,57 Unfortunately, their follow-up of 48 to 52 weeks was inappropriate to evaluate either tissue response or tissue replacement, because little or no signs of material degradation had taken place. Gatzka et al.56 followed a series of patients after stabilization of ankle fractures with high molecular-weight PLLA screws.56 In a study of MRI scans, they found that no osseous replacement of the implant had occurred up to 6 years after implantation (Fig 10 ). Pistner et al.47 studied the intraosseous long-term fate of injection-molded PLLA and PLLA-co-PDLLA screws inserted in the femur of guinea pigs. After implantation of 150 weeks, they found that osseous replacement of the former implant site had occurred and, therefore, stated that amorphous polylactides are fully biodegradable materials. However, even for faster-degrading implants, the process of osseous replacement may require several years if there has been evidence of an osteolytic lesion during the final stage of degradation (Fig 12).
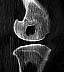
| Figure 12. CT scan 24
months after implantation of a PGA pin in a distal sheep femur. There is still a moderate
osteolytic lesion with no signs of new bone formation, although the implant site contained
no PGA material after 6 months. (Reprinted with permission.46) |
 |
Click on Image to view full size |
| BIOCOMPATIBILITY AND CLINICAL CLASSIFICATION OF TISSUE RESPONSE | TOP |
Since the mid 1960s, many studies have been performed to evaluate the suitability of
various synthetic biodegradable polymers. Prompted by the results arising out of these
investigations, biodegradable implants for various orthopaedic procedures have been
introduced. However, the biocompatibility of these materials is still controversial.
The degradation process and tissue response have been documented by many authors. These
studies show that biodegradable poly--hydroxy acids cause mild, nonspecific tissue
response with fibroblast activation and the invasion of macrophages, multinucleated
foreign-body giant cells, and neutrophilic polymorphonuclear leukocytes during their final
stage of degradation.47,48,51-53,57-62 The initial
euphoria arising out of excellent clinical results was abated by the first reports of
foreign-body reactions with biodegradable implants in fracture treatment. In 1987,
Böstman et al.63 reported a sterile sinus
formation after the use of PGA rods in ankle fractures. Since then, other reports have
shown that foreign-body reactions to PGA implants occurred in varying degrees of severity
ranging from mild osteolytic changes to intense granulomatous inflammatory soft-tissue
lesions necessitating surgical intervention.46,64-68 This reported
intensive inflammatory tissue response was associated with the use of highly crystalline
self-reinforced PGA implants, which consequently led to a decrease in their clinical use.
However, these experiences led to deep concerns about the suitability of biodegradable
implants in orthopaedic surgery.
Many different biodegradable polymers are currently available with better
biocompatibility, such as PDS, PLLA including its stereocopolymers and copolymers, and
some PGA copolymers. Because many factors contribute to biocompatibility and many
different polymers are increasingly implanted, it is essential to have standards to
compare the tissue response in experimental or clinical studies and to discuss these
reactions strictly individualized for the different materials. Literature reviews on
tissue reactions to PGA implants have highlighted the problem of the inability to compare
results because of the lack of a well-defined classification system.16,46 Therefore, we
suggest a standardized classification system based on our previous investigations and
clinical experiences.46,51,66,69,70 Such a tool may enable us to gain more
standardized information on the incidence and severity of tissue reactions in relation to
the choice of polymer, implant design, or anatomic location.
Foreign-body reactions to biodegradable implants should be divided into osseous,
extra-articular, and intra-articular synovial inflammatory soft-tissue responses. In each
group, tissue responses are differentiated into 4 groups according to the severity of
radiological and clinical findings.
Osteolysis
The first reaction at the implant site consists of bone resorption stimulated by the
byproducts released during the degradation, and this is visible as radiolucencies on plain
radiographs and computed tomography (CT) scans (Table 2).
| Osteolysis | Radiological Findings |
| O-0 None | No osteolytic changes visible |
| O-1 Mild | Osteolytic changes at the implant site (osteolysis 1 mm or larger than implant diameter) |
| O-2 Moderate | Cystic-like extended osteolysis (osteolysis 3 mm or larger than implant diameter, Fig 13A) |
| O-3 Severe | Confluence of osteolysis into a resorption cavity (if more than 1 implant is used) |
| O-4 Disturbed healing | Fracture displacement, fragment sequestration, or healing failure of soft tissue due to osteolysis (Fig 13B) |
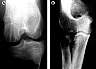
| Figure 13. (A) Cystically
extended resorption cavities (O-2) 12 weeks after osteochondral fragment fixation in a
sheep with self-reinforced PGA pins. (Reprinted with permission.46) (B) Fracture sequestration (O-4) after
stabilization of a multifragmentary radial head fracture with PLLA pins. The fracture
situation has been considered to be unstable, and osteolyses occurred 6 months after
surgery, although final material degradation is expected to occur later. |
 |
Click on Image to view full size |
MRI scans are often appropriate to measure these lesions, but interpretation of findings may be difficult because of the reactive surrounding zone accompanying the final implant degradation.71 Radiolucencies vary from mild osteolytic changes at the implant site to cystic-like extended resorption cavities (Fig 13A ). Mild osteolytic changes probably have no effect on fracture healing, soft-tissue fixation, or the static properties of the bone.71,72 However, if these changes exceed a certain level, they are likely to interfere with fracture healing (Fig 13B )73 or graft fixation. The predictable osteolytic reaction described for PGA implants46,65,68,74-77 has also been observed to be associated with the use of PLLA, PDLLA-co-PGA, PGA-co-TMC, and PLLA stereocopolymers, although with a lower incidence and intensity.51,78-80
Extra-articular Soft-Tissue Reactions
If the material is applied extra-articularly in soft tissue or in cancellous bone of the
metaphysis, such as wrist or ankle fractures or the tibial interference screw in anterior
cruciate ligament reconstruction, the debris accumulated at the implant site during
degradation could be expelled into the surrounding soft tissue (Table 3, Fig 14).
| Extra-articular Soft-Tissue Reactions | Symptoms/Findings/Treatment | |
| EA-0 | None | No or subclinical reaction |
| EA-1 | Mild | Local, mild soft-tissue induration; no treatment |
| EA-2 | Moderate | Fluctuant swelling, fluid accumulation (ultrasound), local warmth, reddening, swelling, pain; single or repetitive puncture necessary (Fig 15A) |
| EA-3 | Severe | Spontaneous discharge of sinus, primary sterile, secondary possible bacterial contamination; debridement and open wound treatment (Fig 15B) |
| EA-4 | Bacterial superinfection | Deep soft-tissue/bone infection following EA-2 or EA-3; extensive and repetitive debridement |
| Figure 15. (A) Subcutaneous
fluctuant swelling (EA-2) after reduction of a Rockwood type V acromioclavicular joint
separation with a PDS band. (B) Spontaneous discharge of debris (EA-3) after stabilization
of a wrist fracture with self-reinforced PGA rods. (Reprinted with permission.69 Copyright 1997 by Springer-Verlag.) |
| |
Click on Image to view full size |
This can be followed by a progressive inflammatory response, manifesting as a subcutaneous soft-tissue induration or a fluctuant swelling that may perforate the skin and form a sinus (Fig 15 ). The incidence depends on the anatomic location and ranges from 4% to 14.6% in ankle fractures and from 22.5% to 40% in wrist fractures if self-reinforced PGA implants are used.66,68,74,81 These reactions have also been observed with a much lower incidence and intensity for PDS or PLLA implants.45,82-85
Intra-articular Synovial Reactions
The intra-articular biocompatibility is of special interest in the field of operative
sports medicine because most implants are applied intra-articularly, such as sutures or
tacks for meniscus or labrum repair, or the implant site may be connected with the joint
space as in the case of interference screws or suture anchors (Table 4).
| Intra-articular Synovial Reactions | Symptoms/Findings/Treatment |
| IA-0 None | No or subclinical reaction |
| IA-1 Mild | Mild (sterile) joint effusion, no additional local or systemic signs of inflammation, single need for puncture, foreign-body giant cells, round cells, or implant remnants in puncture fluid or synovial membrane |
| IA-2 Moderate | Significant (sterile) joint effusion, no other additional local or systemic signs of inflammation, need for recurrent puncture, foreign-body giant cells, round cells, or implant remnants in puncture fluid or synovial membrane; administration of nonsteroidal anti-inflammatory drugs, partial weight-bearing until disappearance of symptoms |
| IA-3 Severe | Significant (sterile) joint effusion with local signs of inflammation (pain, reddening, warmth), need for recurrent punction or surgical revision (e.g., arthroscopic synovectomy), foreign-body giant cells, round cells, or implant remnants in puncture fluid or synovial membrane |
| IA-4 Bacterial superinfection | IA-1 to IA-3 and positive microbiological examination, arthroscopic or open debridement with lavage and synovectomy |
Whereas osteolysis and extra-articular reactions are associated with the final stage of implant degradation, an inflammatory intra-articular response may also be associated with loosened fragments or wear debris released before implant degradation. This has been shown for the knee and shoulder joint86,87 and may occur principally with tacks for labrum or meniscus repair. As soon as a connection between the implant site and the joint space exists, the synovial membrane can come into contact with the polymeric debris at the time of final degradation (Fig 16).
Barfod and Svendsen88 and Friden and
Rydholm89 reported cases of severe synovitis
following intra-articular use of crystalline self-reinforced PGA rods. In these cases,
crystalline polymeric debris surrounded by foreign-body giant cells could be identified as
the cause. Recent reports describe a high incidence of loss of motion with synovial
adhesions attributable to the inflammatory response after the use of PGA-co-TMC tacks in
the shoulder joint.39,90-92 Intra-articular synovial reactions vary from mild
joint effusions to severe synovitis with the necessity of surgical intervention (Table 4
).
As compromised biocompatibility is most commonly detected in the latter stages of implant
decomposition, it is well accepted that the degradation byproducts are responsible for
tissue reactions. Consequently, this implies that a large amount of byproducts being
released per time unit from the implant cannot be adequately handled by the clearing
capacity of the surrounding tissue. This mainly depends on the degradation kinetics of the
implant. This process can last up to several years and influences the time schedule for
experimental or clinical follow-up studies. Maximum extent of foreign-body reactions
associated with PGA implants should occur approximately 12 weeks after surgery.46,57 Those
accompanied with PDS, PGA-co-TMC, or PDLLA-co-PGA may occur between 8 and 24 weeks after
implantation. With the few reported cases of foreign-body reactions associated with PLLA
or PLLA-co-PDLLA implants, they may occur between 1 and 2 years at the earliest but
normally occur later, depending on implant processing techniques, stereocopolymer
composition, implant design, and molecular weight.51,82,85,93
As for soft-tissue reactions, it is reasonable to assume that fast accumulation of implant
fragments or low molecular-weight byproducts cannot be handled adequately by the clearing
capacity of the tissue, represented by macrophages and polymorphonuclear leukocytes.
Therefore, soft-tissue reactions are mostly associated with fast-degrading implants, such
as those composed of PGA. However, they may also be observed for PLLA if the implant
volume exceeds a certain level and the local clearing capacity of the tissue is
overloaded.82
It is known that debris of degradable or nondegradable materials, such as polyethylene or
polymethylmethacrylate, leads to an inflammatory tissue response if the particles get
phagocytosed by macrophages.18,62,94,95 In addition, macrophage activation leads to bone
resorption via mediator release, which results in osteoclast activation.96-98 This may
account for the appearance of osteolytic changes with the use of biodegradable implants,
because maximum macrophage accumulation at the tissue-implant interface correlates with
the maximum expansion of osteolysis, as it has been described for PGA implants.46,57
As an important factor, there are several reports that the local decrease in pH at the
implant site during the degradation is 1 of the main reasons for the inflammatory tissue
response.99-101
On the contrary, in a recent study, Ignatius and Claes102
were able to show that the accumulation of PLLA-co-PDLLA or PLLA-co-PGA degradation
products itself may reduce growth in cell culture. The toxic influence was dependent on a
high concentration of degradation products after pH adjustment.
It is reasonable to assume that a protracted degradation is of primary importance in
increasing the biocompatibility of a biodegradable implant, especially with regard to the
soft-tissue response. But even slow- degrading and amorphous polymers may provoke
osteolytic changes if there is insufficient drainage of byproducts in the surrounding
tissues or when the cellular clearing capacity may be overloaded.
However, other factors appear to contribute to biocompatibility. Matlaga et al.103 and Lam et al.104
showed that even the implant shape affects the intensity of an inflammatory response using
degradable and nondegradable polymers. This has largely been discussed for the
self-reinforcement of PGA implants but has not yet been proved. Additionally, mechanical
instability at the implant site may accelerate degradation and may consequently lead to a
higher amount of degradation products being released per unit of time, thus possibly
increasing the host-tissue response. Furthermore, the crystallinity of a biodegradable
implant, which prevents late hydrolytic degradation, can result in a foreign-body
reaction.44,104-106 Thus, use of materials with low crystallinity
has been advocated for medical purposes.107
Synovial reactions are associated with the release of implant fragments into the joint
space. This rare but severe complication was observed with the use of PGA, PGA-co-TMC, or
PLLA implants in the knee and shoulder joints.39,46,86,88-92,108,109 This
specific synovial reaction to polymeric particles also occurred with a high incidence
using artificial nondegradable ligaments for cruciate ligament reconstruction.110-114 Ligament
wear particles were identified as the cause,115-117 and recent clinical observations and an
experimental study have shown that these wear particles are deposited in the draining
lymph nodes.118,119
This phenomenon has also been described for crystalline PGA and PLLA implants, which
suggests that only incomplete degradation of highly crystalline materials occurs46,120 (Fig 1 ).
Future studies should take into consideration the fact that crystalline implant remnants
may provoke late synovial reactions; for example, if highly crystalline PGA, PLLA, or
PGA-co-TMC implants, such as tacks and pins for labrum and meniscus repair, are used
intra-articularly. The fatal long-term results of these reactions after stabilization of
ankle fractures with PGA rods has recently been described.108
Böstman108 reported the development of a
moderate to severe osteoarthritis of the ankle that occurred 36 to 109 months after
surgery in 10 of 74 patients who had previous inflammatory soft-tissue reactions. He
concluded that the joint damage seemed to be caused by polymeric debris entering the
articular cavity through an osteolytic lesion.
| CONCLUSION | TOP |
The use of biodegradable implants offers distinct advantages in the field of operative
sports medicine. Thus, research and development of biodegradable implants should be given
high priority. The research on these devices should be encouraged by the will to define
and solve problems and to find technical solutions, rather than driven by the desire for
quick results.
Concerns about the poor biocompatibility of self-reinforced PGA implants do not
necessarily apply to other materials with an appropriate tissue response. Biocompatibility
depends on a large variety of factors. Therefore, each biodegradable implant should be
tested regarding its intraosseous, soft-tissue, and intra-articular biocompatibility, and
discussion of the results should be strictly individualized for each of the different
polymers, copolymers, and stereocopolymers. Furthermore, in vivo long-term studies are
necessary, with follow-up until implant remnants have disappeared and an osseous
replacement has taken place. To gain more information on biocompatibility according to the
specific choice on polymer and implantation site, the clinical use of biodegradable
implants is recommended to be performed under study conditions, and all results concerning
tissue response should be evaluated with a standardized classification system.
| REFERENCES | TOP |
1. Shellock FG, Mink JH, Curtin S, Friedman MJ. MR imaging and metallic implants for anterior cruciate ligament reconstruction: Assessment of ferromagnetism and artifact. J Magn Reson Imaging 1992;2:225-228.
|
|
3. Disegi JA, Wyss H. Implant materials for
fracture fixation: A clinical perspective. Orthopedics 1989;12:75-79.
|
4. Rehm KE, Helling HJ, Claes LE. Biologisch
abbaubare Osteosynthesematerialien. In: Bünte H, Jungiger T, eds. Jahrbuch der
Chirurgie. Zülpich, Germany: Biermann Verlag, 1989;223-232.
5. Weiler A, Windhagen H, Raschke MJ, Laumeyer
A, Hoffmann RFG. Biodegradable interference screw fixation exhibits pull-out force and
stiffness similar to titanium screws. Am J Sports Med 1998;26:119-128.
|
6. Caborn D, Urban WP, Johnson DL, Nyland J,
Pienkowski D. Biomechanical comparison between BioScrew and titanium alloy interference
screws for bone–patellar tendon–bone graft fixation in anterior cruciate
ligament reconstruction. Arthroscopy 1997;13:229-232.
|
|
7. Rupp S, Krauss PW, Fritsch EW. Fixation
strength of a biodegradable interference screw and press-fit technique in anterior
cruciate ligament reconstruction with a BPTB graft. Arthroscopy 1997;13:61-65.
|
|
8. Barber FA, Herbert MA, Click MA. Suture
anchor strength revisited. Arthroscopy 1996;12:32-38.
|
|
9. Barber FA, Herbert MA, Click JN. The
ultimate strength of suture anchors. Arthroscopy. 1995;11:21-28.
|
|
10. Claes LE. Mechanical characterization of
biodegradable implants. Clin Mater 1992;10:41-46.
|
11. Daniels AU, Chang MKO, Andriano KP.
Mechanical properties of biodegradable polymers and composites proposed for internal
fixation of bone. J Appl Biomater 1990;1:57-78.
|
12. Higashi S, Yamamuro T, Nakamura T, Ikada
Y, Hyon SH, Jamshidi K. Polymer-hydroxyapatite composites for biodegradable bone fillers. Biomaterials
1986;7:183-187.
|
13. Heidemann W, Gerlach KL, Fischer JH,
Ruffieux K, Wintermantel E, Jeschkeit S. Tissue reaction to implantation of
poly(D,L)lactide with or without addition of calciumphosphates in rats. Biomed Tech
1996;41:408-409 (suppl 1).
14. Fischer JH, Ruffieux K, Jeschkeit S,
Heidemann W, Gerlach KL, Wintermantel E. In vivo versus in vitro evaluation of
poly(D,L)lactide rods including calcium phosphate particles. Presented at the
International Symposium on Biodegradable Materials, Hamburg, 1996.
15. Prokop A, Helling HJ, Fischbach R,
Wollsiefer M, Dietershagen M, Reif D, Rehm KE. Neue biodegradierbare
Tricalciumphosphat-Polylactidstifte zur Refixation osteochondraler Fragmente. Erste
radiologische Ergebnisse einer tierexperimentellen Untersuchung. Presented at the 3rd
European Trauma Congress, Amsterdam, 1998.
16. Athanasiou KA, Niederauer GG, Agrawal CM.
Sterilization, toxicity, biocompatibility and clinical applications of polylactic
acid/polyglycolic acid copolymers. Biomaterials 1996;17:93-102.
|
17. Hollinger JO, Battistone GC. Biodegradable
bone repair materials. Synthetic polymers and ceramics. Clin Orthop
1986;207:290-305.
|
18. Lam KH, Schakenrad JM, Esselbrugge H,
Feijen J, Nieuwenhuis P. The effect of phagocytosis of poly(L-lactic acid) fragments on
cellular morphology and viability. J Biomed Mater Res 1993;27:1569-1577.
|
19. Tabata Y, Ikada Y. Macrophage phagocytosis
of biodegradable microspheres composed of L-lactic acid/glycolic acid homo- and
copolymers. J Biomed Mater Res 1988;22:837-858.
|
20. Chu CC. Scanning electron microscopic
study of the hydrolytic degradation of poly(glycolic acid) sutures. J Biomed Mater Res
1982;16:417-430.
|
21. Williams F, Mort E. Enzyme-accelarated
hydrolysis of polyglycolic acid. J Bioengen 1977;1:231-238.
22. Zhang X, Wyss UP, Pichora D, Goosen FA. An
investigation of poly(lactic acid) degradation. J Bioac Comp Pol 1994;9:80-100.
23. David A, Eitenmüller J, von Oepen R,
Müller D, Pommer A, Muhr G. [Mechanical and chemical stability of biodegradable
block-polymerized and injection-molded poly-L-lactic acid in vitro]. Unfallchirurg
1994;97:278-284.
|
24. Gerlach KL, Eitenmüller J, Schmitz H. [In
vivo study of the strength properties of biodegradable polymers for application as
osteosynthesis materials]. Dtsch Z Mund Kiefer Gesichtschir 1987;11:211-216.
|
25. Gogolewski S. Bioresorbable internal
fixation devices—Mechanical properties and future trends in production technologies.
Presented at the meeting of the International Society for Fracture Repair, Hong Kong,
1993.
26. Leenslag JW, Pennings AJ, Bos RRM, Rozema
FR, Boering G. Resorbable materials of poly(L-lactide). VI. Plates and screws for internal
fracture fixation. Biomaterials 1987;8: 70-73.
|
27. Mainil-Varlet P, Rahn B, Gogolewski S.
Long-term in vivo degradation and bone reaction to various polylactides. One-year results.
Biomaterials 1997;18:257-266.
|
28. Dauner M, Hierlemann H, Müller E, Planck
H. Degradation verschiedener Strukturen aus resorbierbaren Polymeren. In: Claes L,
Ignatius A, eds. Biodegradierbare Implantate und Materialien. Berlin:
Springer-Verlag, 1998;75-82.
29. Rozema FR, van Asten JAAM, Bos RRM,
Boering G, Cordewener FW, Nijenhuis AJ, Pennings AJ. The effects of different
steam-sterilization programmes on material properties of poly(L-lactide). Presented at the
Fourth World Biomaterials Congress, Berlin, 1992.
30. Pistner H, Stallforth H, Gutwald R,
Mühling J, Reuther J, Michel C. Poly(L-lactide): A long term study in vivo. Part II:
Physico-mechanical behaviour of implants. Biomaterials 1994;15:439-450.
|
31. Vert M, Christel P, Chabot F, Leray J.
Bioresorbable plastic materials for bone surgery. In: Hastings GW, Ducheyne P, eds. Macromolecular
biomaterials. Boca Raton, FL: CRC, 1984;120-142.
32. Mainil-Varlet P, Cordey J, Gogolewski S.
Positional stability of polylactide pins with various surface texture in the sheep tibia. J
Biomed Mater Res 1997;34:351-359.
|
33. Liew A, Johnson D. Efficacy of
bioabsorbable interference fit screws for hamstring fixation in ACL reconstruction.
Presented at the 18th Annual Meeting of the Arthroscopy Association of North America,
Vancouver, 1998.
34. Stähelin AC, Feinstein R, Friedrich NF.
Clinical experience using a bioabsorbable interference screw for ACL reconstruction. Orthop
Trans 1995;19:287-288.
35. Toljan MA, Orthner E, Reichel M. Bone
block fixation with resorbable interference screws. An MRI and immunhistochemical study.
Presented at the 6th Congress of the European Society of Sports Traumatology, Knee
Surgery, and Arthroscopy, Berlin, 1994.
36. Stähelin AC, Weiler A, Rüfenacht H,
Hoffmann R, Geissmann A, Feinstein R. Clinical degradation and biocompatibility of
different bioabsorbable interference screws: A report of six cases. Arthroscopy
1997;13:238-244.
|
|
37. Arciero RA, Taylor DC, Snyder RJ, Uhorchak
JM. Arthroscopic bioabsorbable tack stabilization of initial anterior shoulder
dislocations: A preliminary report. Arthroscopy 1995;11:410-417.
|
|
38. Speer K, Warren RF. Arthroscopic shoulder
stabilization—A role for biodegradable materials. Clin Orthop 1993;291: 67-74.
|
39. Warner J, Miller M, Marks P, Fu F.
Arthroscopic Bankart repair with the Suretac device. Part I: Clinical observation. Arthroscopy
1995;11:2-13.
|
|
40. Weiler A, Peine R, Pashmineh-Azar R,
Unterhauser F, Hoffmann RFG. Tendon to bone healing under direct interference screw
fixation in a sheep model. Arthroscopy 1998;14:437-438.
41. Champion AR, Cutshall TA, van Sicke DC. In
vitro and vivo evaluation of a bioresorbable interference screw. Presented at the 41st
Annual Meeting of the Orthopaedic Research Society, Orlando, 1995.
42. Therin M, Chambat P, Fayar JP, Christel P.
In vivo evaluation of bioabsorbable interference screws (98% PLLA, 2% PDLLA) in sheep.
Presented at the 7th Congress of the European Society of Sports Traumatology, Knee
Surgery, and Arthroscopy, Budapest, 1996.
43. Walton M, Cameron M. Efficacy of an
absorbable interference screw for graft fixation in anterior cruciate ligament
reconstruction: A study using a sheep model. J Bone Joint Surg Br 1996;78:126
(suppl II & III).
44. Bergsma EJ, de Bruijn WC, Rozema FR, Bos
RRM, Boering G. Late degradation tissue response to poly(L-lactide) bone plates and
screws. Biomaterials 1995;16:25-31.
|
45. Böstman O, Pihlajamäki H, Partio E,
Rokkanen P. Clinical biocompatibility and degradation of polylevolactide screws in the
ankle. Clin Orthop 1995;320:101-109.
|
46. Weiler A, Helling HJ, Kirch U, Zirbes TK,
Rehm KE. Foreign-body reactions and the course of osteolysis after polyglycolide implants
for fracture fixation: Experimental study in sheep. J Bone Joint Surg Br
1996;78:369-376.
|
47. Pistner H, Reuther J, Mühling J, Gutwald
R. Vollständige Biodegradation von amophen Polylactid-Osteosynthesematerialien in Hart-
und Weichgewebe im Langzeitversuch. In: Oester HJ, Rehm KE, eds. 61st Jahrestagung der
Deutschen Gesellschaft für Unfallchirurgie. Berlin: Springer-Verlag, 1997; 756-766.
48. Vainionpää S. Biodegradation of
polyglycolic acid in bone tissue: An experimental study on rabbits. Arch Orthop Trauma
Surg 1986;104:333-338.
|
49. Majola A. Fixation of experimental
osteotomies with absorbable polylactic acid screws. Ann Chir Gynaecol
1991;80:274-281.
|
50. Majola A, Vainionpää S, Vihtonen K,
Vasenius J, Törmälä P, Rokkanen P. Intramedullary fixation of cortical bone osteotomies
with self-reinforced polylactic rods in rabbits. Int Orthop 1992;16:101-108.
|
51. Helling HJ, Kirch U, Weiler A, Rehm KE.
Zelluläre Reaktionen während des Abbaus von Polylactid PL/DLLA 70/30. Bioresorbierbare
Implantatmaterialien: Symposium der Deutschen Gesellschaft für Biomaterialien, Günzburg,
1996.
52. Böstman OM, Päivärinta U, Partio E,
Manninen M, Vasenius J, Majola A, Rokkanen P. The tissue-implant interface during
degradation of absorbable polyglycolide fracture fixation screws in the rabbit femur. Clin
Orthop 1992;285:263-272.
|
53. Nordström P, Pihlajamäki H, Toivonen T,
Törmälä P, Rokkanen P. Tissue response to polyglycolide and polylactide pins in
cancellous bone. Arch Orthop Trauma Surg 1998;117:197-204.
|
54. Lajtai G, Balon R, Humer K, Aitzetmüller
G, Unger F, Orthner E. Resorbierbare Interferenzschrauben: Histologische Untersuchung 4,5
Jahre postopertiv—Eine Kasuistik. Unfallchirurg 1998;102:866-869.
55. Pistner H, Gutwald R, Ordung R, Reuther J,
Mühling J. Poly(L-lactide): A long-term degradation study in vivo. Part I: Biological
results. Biomaterials 1993;14:671-677.
|
56. Gatzka C, Helling HJ, Prokop A, Fischbach
R, Rehm KE. Metallschrauben versus biodegradierbare
Polylactid-L-Schrauben—Langzeitergebnisse einer prospektiv randomisierten Studie. In:
Oester HJ, Rehm KE, eds. 61st Jahrestagung der Deutschen Gesellschaft für
Unfallchirurgie. Berlin: Springer-Verlag, 1997;766-769.
57. Päivärinta U, Böstman O, Majola A,
Toivonen T, Törmälä P, Rokkanen P. Intraosseous cellular response to biodegradable
fracture fixation screws made of polyglycolide or polylactide. Arch Orthop Trauma Surg
1993;112:71-74.
|
58. Rehm KE, Schultheis KH. [Transposition of
ligaments with polydioxanone (PDS)]. Unfallchirurg 1985;11:264-273.
59. Bos RR, Rozema FR, Boering G, Nijenhuis
AJ, Pennings AJ, Verwey AB, Nieuwenhuis P, Jansen HW. Degradation of and tissue reaction
to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in
rats. Biomaterials 1991;12:32-36.
|
60. Pistner H, Bendix R, Mühling J, Reuther
F. Poly(L-lactide): A long term study in vivo. Part III. Analytical characterization. Biomaterials
1993;14:291-298.
|
61. Matlaga BF, Salthouse TN. Ultrastructural
observations of cells at the interface of a biodegradable polymer: Polyglactin 910. J
Biomed Mater Res 1983;17:185-197.
|
62. Anderson J, Miller K. Biomaterial
biocompatibility and the macrophage. Biomaterials 1985;2:171-176.
63. Böstman O, Vainionpää S, Hirvensalo E,
Mäkelä A, Vihtonen K, Törmälä P, Rokkanen P. Biodegradable internal fixation for
malleolar fractures. A prospective randomised trial. J Bone Joint Surg Br
1987;69:615-619.
|
64. Poigenfürst J, Leixnering M, Mokhtar MB.
[Local complications after implantation of Biorod]. Akt Traumatol 1990;20:157-159.
65. Böstman O. Osteolytic changes
accompanying degradation of absorbable fracture fixation implants. J Bone Joint Surg Br
1991;73:679-682.
|
66. Hoffmann R, Krettek C, Hetkamper A, Haas
N, Tscherne H. [Osteosynthesis of distal radius fractures with biodegradable fracture
rods. Results of two years follow-up]. Unfallchirurg 1992;95:99-105.
|
67. Böstman O. Intense granulomatous
inflammatory lesions associated with absorbable internal fixation devices made of
polyglycolide in ankle fracture. Clin Orthop 1992;278:191-199.
68. Casteleyn PP, Handelberg F, Haentjens P.
Biodegradable rods versus Kirschner wire fixation of wrist fractures. A randomised trial. J
Bone Joint Surg Br 1992;74:858-861.
|
69. Hoffmann R, Weiler A, Helling HJ, Krettek
C, Rehm KE. [Local foreign-body reactions to biodegradable implants. A classification]. Unfallchirurg
1997;100:658-666.
|
70. Hoffmann R, Krettek C, Haas N, Tscherne H.
[Distal radius fracture. Fracture stabilization with biodegradable osteosynthesis pins
(Biofix). Experimental studies and initial clinical experiences]. Unfallchirurg
1989;92:430-434.
|
71. Lajtai G, Noszian I, Humer K, Unger F,
Aitzetmüller G, Orthner E. Serial MRI evaluation of operative site following fixation of
patellar tendon graft with bioabsorbable interference screws in ACL reconstruction.
Personal communication, 1998.
72. Weiler A, Helling HJ, Kirch U, Rehm KE.
Tierexperimentelle Langzeituntersuchung über Fremdkörperreaktionen und Osteolysen nach
Verwendung von Polyglykolidimplantaten. In: Cleas L, Ignatius A, eds. Biodegradierbare
Implantate und Materialien. Berlin: Springer-Verlag, 1997;146-159.
73. Svensson PJ, Janarv PM, Hirsch G. Internal
fixation with biodegradable rods in pediatric fractures: One-year follow-up of fifty
patients. J Pediatr Orthop 1994;14:220-224.
|
74. Frokjaer J, Moller BN. Biodegradable
fixation of ankle fractures. Complications in a prospective study of 25 cases. Acta
Orthop Scand 1992;63:434-436.
|
75. Gerbert J. Effectiveness of absorbable
fixation devices in Austin bunionectomies. J Am Podiatr Med Ass 1992;82:189-195.
76. Fraser RK, Cole WG. Osteolysis after
biodegradable pin fixation of fractures in children. J Bone Joint Surg Br
1992;74:929-930.
|
77. Lavery LA, Peterson JD, Pollack R, Higgins
KR. Risk of complications of first metatarsal head osteotomies with biodegradable pin
fixation: Biofix versus Orthosorb. J Foot Ankle Surg 1994;33:334-340.
|
78. Suuronen R. Biodegradable
fracture-fixation devices in maxillofacial surgery. Int J Oral Maxillofac Surg
1993;22:50-57.
|
79. DeBerardino TM, Arciero RA, Uhorchak JM,
Taylor DC. Long-term radiographic analysis of absorbable and non-absorbable implants used
in Bankart repairs. Presented at the 17th Annual Meeting of the Arthroscopy Association of
North America, Orlando, 1998.
80. Lajtai G, Humer K, Unger F, Aitzetmüller
G, Noszian I, Orthner E. Bioabsorbable interference screws for ACL reconstruction: A new
material, an expanded clinical assessment. Personal communication, 1998.
81. Hirvensalo E. Fracture fixation with
biodegradable rods. Forty-one cases of severe ankle fractures. Acta Orthop Scand
1989;60:601-606.
|
82. Eitenmüller J, David A, Pommer A, Muhr G.
[Internal fixation of ankle fractures with biodegradable poly-L-lactide screws and
plates]. Chirurg 1996;67:413-418.
|
83. Hofmann GO. Biodegradable implants in
traumatology: A review on the state-of-the-art. Arch Orthop Trauma Surg
1995;114:123-132.
|
84. Kalla TP, Janzen DL. Orthosorb: A case of
foreign-body reaction. J Foot Ankle Surg 1995;34:366-370.
|
85. Böstman O, Pihlajamäki H. Late
foreign-body reaction to an intraosseous bioabsorbable polylactide acid screw. J Bone
Joint Surg Am 1998;80:1791-1794.
|
86. Takizawa T, Akizuki S, Horiuchi H,
Yasukawa Y. Case report. Foreign-body gonitis caused by a broken poly-L-lactic acid screw.
Arthroscopy 1998;14:329-330.
|
|
87. Kurzweil PR, Schreck PJ. Meniscus fixation
using the arrow in human and goat knees. Presented at the 17th Annual Meeting of the
Arthroscopy Association of North America, Orlando, 1998.
88. Barfod G, Svendsen RN. Synovitis of the
knee after intraarticular fracture fixation with Biofix. Report of two cases. Acta
Orthop Scand 1992;63:680-681.
|
89. Friden T, Rydholm U. Severe aseptic
synovitis of the knee after biodegradable internal fixation. Acta Orthop Scand
1992;63:94-97.
|
90. Bennett WF. Bioabsorbable soft tissue
fasteners: Failure mode an exaggerated inflammatory response? Presented at the 17th Annual
Meeting of the Arthroscopy Association of North America, Orlando, 1998.
91. Edwards D, Hoy G, Saies A, Hayes M.
Adverse reactions to an absorbable shoulder fixation device. J Shoulder Elbow Surg
1994;3:230-233.
92. Imhoff A, Burkart A, Roscher E. Adverse
reactions to bioabsorbable Suretac device in arthroscopic shoulder stabilization and
SLAP-refixation. Presented at the 8th Congress of the European Society of Sports Medicine,
Knee Surgery, and Arthroscopy, Nice, 1998.
93. Helling HJ, Weiler A, Kirch U, Rehm KE.
Experimental use of a new biodegradable polylactide-pin for the refixation of
osteochondral fragments—And first clinical experiences. Presented at the 6th Congress
of the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy, Berlin,
1994.
94. Horowitz SM, Gautsch TL, Frondoza CG,
Riley L. Macrophage exposure to polymethylmethacrylate leads to mediator release and
injury. J Orthop Res 1991;7:290-305.
95. Greisler HP. Bioresorbable materials and
macrophage interactions. J Vasc Surg 1991;13:748-750.
|
96. Klein DC, Raisz LG. Prostaglandins:
Stimulation of bone resorption in tissue culture. Endocrinology 1970;86:1436-1440.
|
97. Cohn ZA. The activation of mononuclear
phagocytes: Fact, fancy, and future. J Immunol 1978;121:813-816.
|
98. Minkin C, Shapiro IM. Osteoclasts,
mononuclear phagocytes, and physiological bone resorption. Calcif Tissue Int
1986;39:357-359.
|
99. Daniels AU, Taylor MS, Andriano KP, Heller
J. Toxicity of absorbable polymers proposed for fracture fixation devices. Presented at
the 38th Annual Meeting of the Orthopaedic Research Society, San Francisco, 1992.
100. Suganuma J, Alexander H. Biological
response of intramedullary bone to poly-L-lactic acid. J Appl Biomater 1993;4:
13-27.
101. Agrawal CM, Athanasiou KA. A technique
to control the pH in the vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res
1997;38:105-114.
|
102. Ignatius AA, Claes LE. In vitro
biocompatibility of bioresorbable polymers: Poly(L,DL-lactide) and
poly(L-lactide-co-glycolide). Biomaterials 1996;17:831-839.
|
103. Matlaga BF, Yasenchak LP, Salthouse TN.
Tissue response to implanted polymers: The significance of sample shape. J Biomed Mater
Res 1976;10:391-397.
|
104. Lam KH, Schakenraad JM, Esselbrugge H,
Dijkstra PJ, Feijen J, Nieuwenhuis P. Quantitative biocompatibility of biodegradable
polymers as studied by physico-chemical and cell biological parameters. In: Doherty PJ,
ed. Biomaterial—Tissue interfaces. Amsterdam: Elsevier, 1992;43-48.
105. Rozema FR, de Bruijn WC, Bos RRM,
Boering G, Nijenhuis AJ, Pennings AJ. Late tissue response to bone-plates and screws of
poly(L-lactide) used for fracture fixation of the zygomatic bone. In: Editor? Biomaterial—Tissue
interfaces. Amsterdam: Elsevier, 1992;349-355.
106. Bergsma EJ, Rozema FR, Bos RRM, de
Bruijn WC. Foreign body reactions to resorbable poly(L-lactide) bone plates and screws
used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg
1993;51:666-670.
|
107. Andriano KP, Pohjonen T, Törmälä P.
Processing and characterization of absorbable polylactide polymers for use in surgical
implants. J Appl Biomater 1994;11:537-548.
108. Böstman OM. Osteoarthritis of the ankle
after foreign-body reaction to absorbable pins and screws—A three- to nine-year
followup study. J Bone Joint Surg Br 1998;80:333-338.
|
109. Tegnander A, Engebretsen L, Bergh K,
Eide E, Holen KJ, Iversen OJ. Activation of the complement system and adverse effects of
biodegradable pins of polylactic acid (Biofix) in osteoarthritis dissecans. Acta Orthop
Scand 1994;65:472-475.
|
110. Paulos LF, Rosenberg JD, Grewe SR. The
Gortex anterior cruciate ligament prosthesis: A long-term follow-up. Presented at the 57th
Annual Meeting of the American Academy of Orthopaedic Surgeons, New Orleans, 1990.
111. Lukianov AV, Richmond JC, Barret GR,
Gillquist J. A multicenter study on the results of anterior cruciate ligament
reconstruction using Dacron ligament prosthesis in “salvage” cases. Am J
Sports Med 1989;17:380-386.
|
112. Jenson K, Klein W. Probleme und
Komplikationen beim künstlichen Kreuzbandersatz. Arthroskopie 1990;3:15-23.
113. Klein W, Jenson K. Synovitis and
artificial ligaments. Arthroscopy 1992;8:116-124.
|
|
114. Roth J, Shkrum M, Bray R. Synovial
reaction associated with disruption of polypropylene braid-augmented intraarticular
anterior cruciate ligament reconstruction: A case report. Am J Sports Med
1988;16:301-305.
|
115. Greis PE, Georgescu HI, Fu FH, Evans CH.
Particle-induced synthesis of collagenase by synovial fibroblasts: An immunocytochemical
study. J Orthop Res 1994;12:286-293.
|
116. Claes LE, Ludwig J, Margevicius KJ,
Dürselen L. Biological response to ligament wear particles. J Appl Biomater
1995;6:35-41.
|
117. Olson EJ, Kang JD, Fu FH. The
biomechanical and histological effects of artificial ligament wear particles: In vitro and
in vivo studies. Am J Sports Med 1988;16:558-570.
|
118. Margevicius KJ, Claes LE, Dürselen L,
Hanselmann KF. Identification and distribution of synthetic ligament wear particles in
sheep. J Biomed Mater Res 1996;31:319-328.
|
119. Plessas SJ, Wilson AG, Forster IW.
Lymphadenopathy after Goretex cruciate reconstruction. Presented at the 7th Congress of
the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy, Budapest,
1996.
120. Verheyen CC, de Wijn JR, van
Blitterswijk CA, Rozing PM, de Groot K. Examination of efferent lymph nodes after 2 years
of transcortical implantation of poly(L-lactide) containing plugs: A case report. J
Biomed Mater Res 1993;27:1115-1118.
|
| Publishing and Reprint Information | TOP |